
Overview
Our lab’s research utilizes the nematode C. elegans as a model system for studying the cell biology and developmental roles of the apical extracellular matrix (aECM). This matrix fills and shapes tube lumens, protects the animal from pathogens and other environmental insults, and forms complex structures that decorate the animal’s surface.

Apical Extracellular Matrix
Most tubes secrete various proteoglycans, glycoproteins and lipoproteins into their developing lumens. Examples in mammals include the vascular glycocalyx, lung surfactant, and the mucus-rich linings of the gut and upper airway. There is a growing appreciation of the importance of this luminal aECM in development and disease. However, aECMs are very difficult to visualize and study in most systems because they are transparent by light microscopy and destroyed by most standard fixation approaches used for immunofluorescence.
We’ve identified components of an early C. elegans aECM that shapes developing epithelia, including tubes of various sizes (left). Many of these components belong to conserved protein families also found in mammalian ECMs. We can visualize these components in live worms using fluorescent tags inserted into the endogenous loci. Our foundational studies have shown that this matrix is extremely complex and dynamic, and that different cell types produce and assemble different types of matrix. Some matrix proteins form complex substructures such as luminal cones or ridges on epidermal surfaces. We are studying how the various components traffic to their correct locations and assemble to form these beautiful patterns.
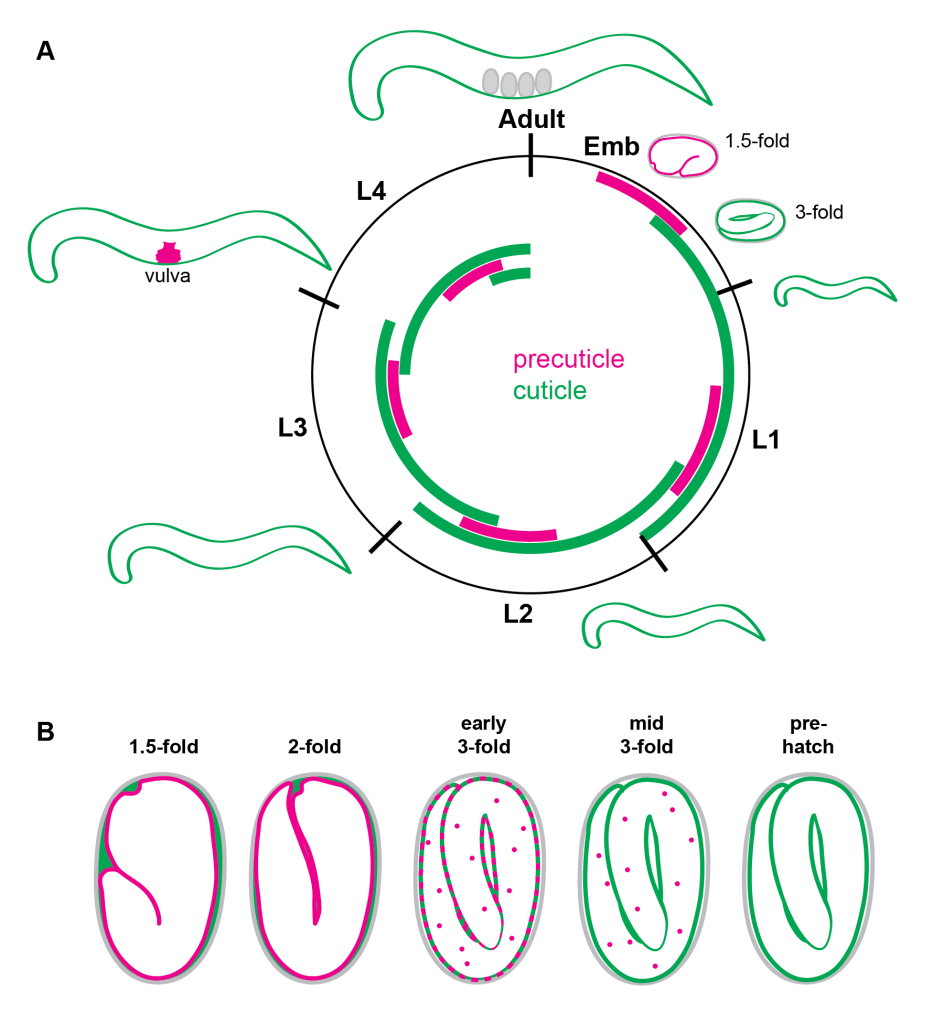
The Pre-cuticle and Cuticle: Dynamic Apical Extracellular Matrices
The precuticle is a transient aECM that precedes each new cuticle during the molt cycle and plays important roles in tube and body shaping, as well as in patterning the next cuticle and shedding of the old one. It contains many Zona Pellucida (ZP) domain proteins, extracellular leucine-rich repeat only (eLRRon) proteins, and Lipocalins. https://pubmed.ncbi.nlm.nih.gov/32975517/
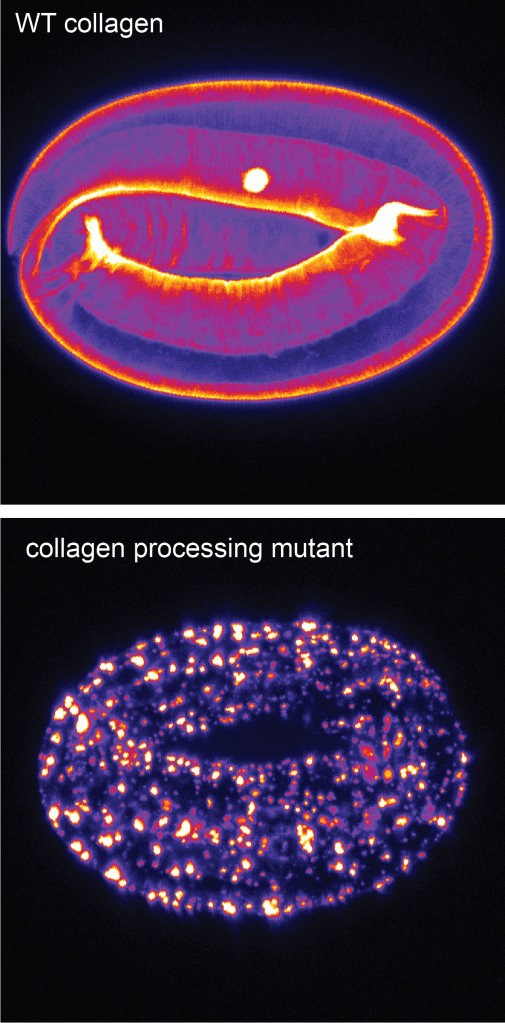
How does proteolysis influence collagen trafficking and assembly?
C. elegans external epithelia eventually become coated by a cuticle or exoskeleton that is primarily made up of collagens. We are beginning to study the proteolytic processing and maturation of these collagens during assembly of the first cuticle and the pre-cuticle to cuticle transition. We take advantage of the fact that it is very easy to see when something goes wrong in the embryo!
https://pubmed.ncbi.nlm.nih.gov/37721936/
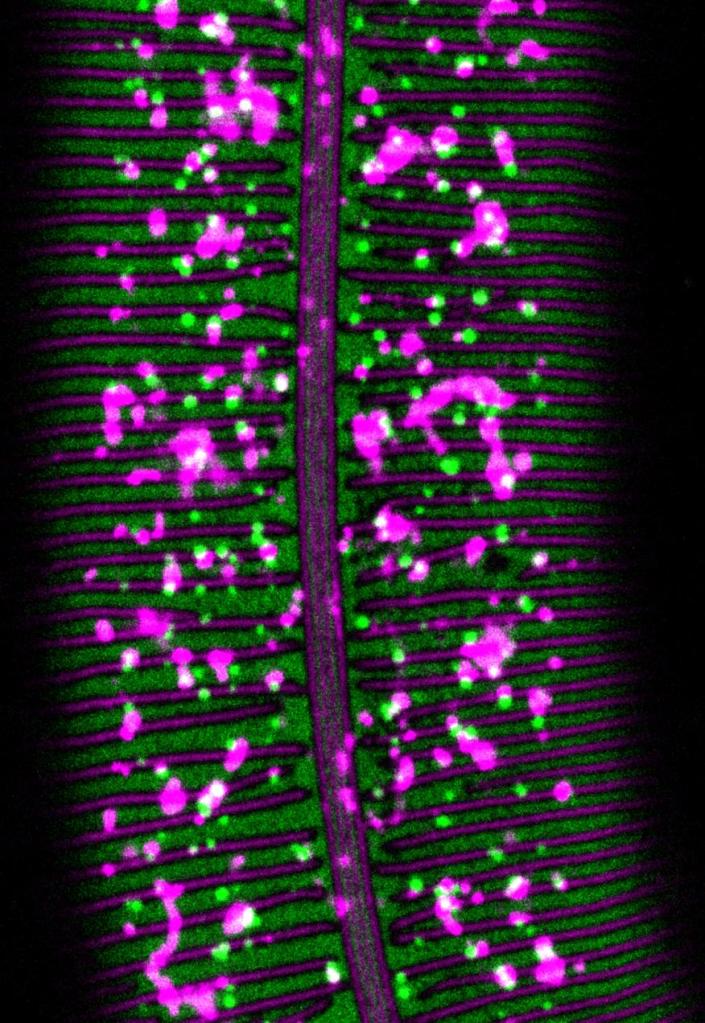
What determines cell- and substructure-specific patterns of matrix assembly?
apical ECM assembles in exquisite patterns, with some broadly expressed proteins binding to only specific cell surfaces or substructures such as annular bands vs. furrows or alae ridges vs. intervening valleys. We are interested in the domains and interactions that drive these patterns of assembly.
https://pubmed.ncbi.nlm.nih.gov/38739761/
https://pubmed.ncbi.nlm.nih.gov/39489317/
Green: WRT-10 Magenta: GRL-7
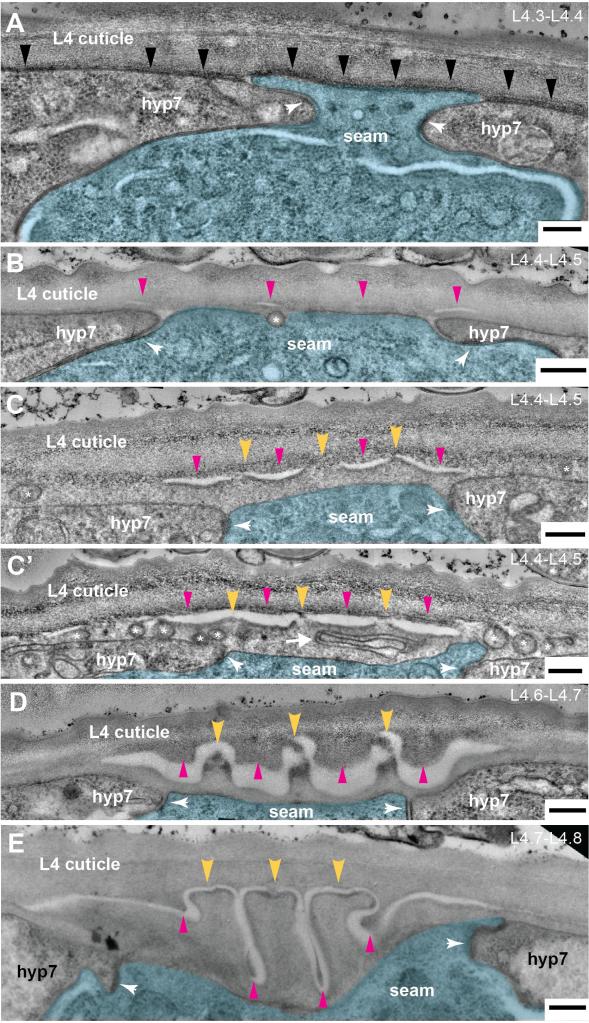
How can matrix structures be shaped in the extracellular environment?
We are studying formation of adult cuticle ridges or “alae” to address this question. At left is an electron microsopy time series showing matrix delamination as one of the initial steps of alae patterning. This delamination is triggered by the underlying actin cytoskeleton. https://pubmed.ncbi.nlm.nih.gov/35960773/